A group of scientists from the University of Maryland in the United States and the East China University of Science and Technology has fabricated a reversible chlorine redox flow battery for stationary energy storage.
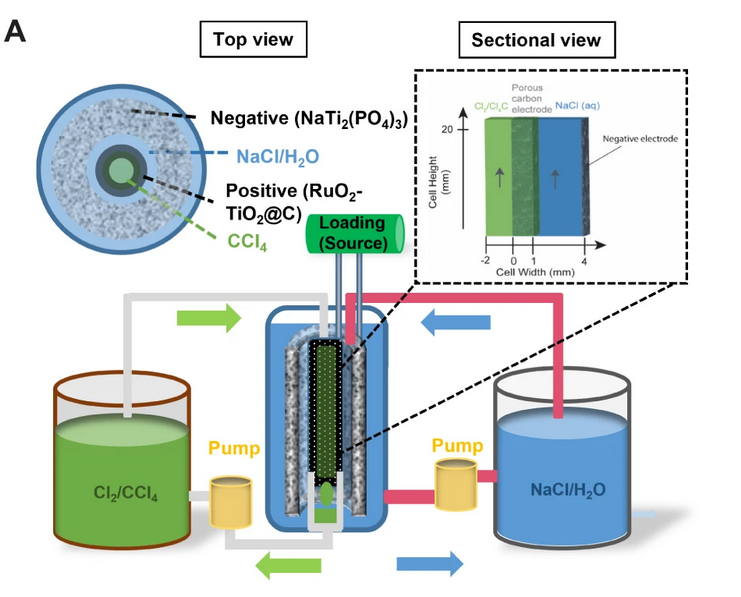
The full chlorine device has a membrane-free design and is based on an aqueous electrolyte made of sodium chloride (NaCl) which uses chlorine (Cl2/Cl−) redox couple as the active material for the positive electrode. “The Cl2/Cl− has a theoretical capacity of 755 mAh/g, more than two times that of vanadium oxides (VO2+/VO2+, 226 mAh/g) used in current redox flow batteries,” the researchers explained. “In addition, sodium chloride is one of the cheapest commodities available due to the abundant source in seawater and large-scale production.”
One of the major costs in vanadium redox flow batteries is the ion-permeable membrane used to minimize so-called “crossover.” This phenomenon, which can cause capacity losses that can reach up to 50%, occurs during charging and recharging, when battery electrolyte components cross the membrane in the battery cell and the redoxmers – which are redox-active molecules that can store energy in the batteries' electrolytes – migrate to the wrong side of the device. In the battery configuration proposed by the US-Chinese group, the chlorine is immiscible to the electrolyte, which means that no membrane to prevent crossover is needed, thus further reducing costs.
The Cl2/Cl− redox reaction in the aqueous NaCl electrolyte was evaluated in a concentric cell with a porous carbon coated with ruthenium(IV) oxide and titanium oxide (RuO2-TiO2), which is intended at promoting the oxidation kinetics of chloride. Carbon tetrachloride (CCl4) was also added through the working electrode and the electrolyte. A graphene composite that is known as NaTi2(PO4)3 was used as the negative electrode.
The battery cell was found to have a high voltage efficiency, which the scientists attributed not only to the fast reaction kinetics but also the membrane-free configuration. “At the highest flow rate examined, the voltage efficiency could be postulated to over 93% at 20 mA/cm2,” they emphasized.
The battery showed a round-trip energy efficiency of 91% at 10 mA/cm2 and an energy density of 125.7 Wh/L. The values, according to the academics, represent the best performance for a flow battery system in the past ten 10 years.
The battery was presented in the study High-energy and low-cost membrane-free chlorine flow battery, published in nature communications. “The chlorine flow battery can meet the stringent price and reliability target for stationary energy storage with the inherently low-cost active materials (around $5/kWh) and the highly reversible Cl2/Cl− redox reaction,” the research team concluded.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.